Effect of H2 on Blast Furnace Smelting
1. Effect of H2 on Reduction Mechanism of Iron-Bearing Burden
After the hydrogen-rich smelting of BF, the reduction behavior of the sinter and pellets in the lump zone changed, and then the property of reduction disintegration changed.The fundamental reason for reduction disintegration is that hematite (Fe2O3) in the iron ore reduces to magnetite (Fe3O4) under low-temperature conditions, resulting in lattice transformation and volume expansion, causing lattice distortion to generate internal stress and fracture and disintegration. The disintegration mechanism is shown in Figure 1.
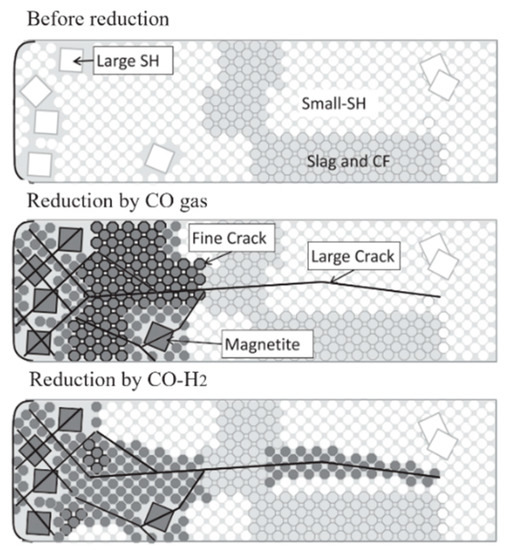
Figure 1. Reduction disintegration mechanism of sinter, adopted from [32].SH: Skeletal hematite;CF: multi-component calcium ferrite.
Since the molecular size of H2 and H2O is much smaller than that of CO and CO2, the reactants and products are more likely to diffuse to or leave the reaction interface in the pores of the iron ore. It can be seen from the kinetic model of the gas-solid reaction that diffusion is the limiting link of the reaction at higher temperatures. After the volume fraction of H2 increases, the reducing gas is more likely to diffuse to the iron ore surface, and at the same time, it is more likely to diffuse through the porous reduction product layer and then to the reduction reaction interface. Therefore, the internal diffusion resistance relatively decreases, and the chemical reaction resistance relatively increases.
After hydrogen-rich smelting in BF, the reduction of iron oxide is accelerated, and the mineral composition and microstructure at various temperatures are changed. Also, a good iron phase appears in the sinter, which is interconnected in a network and has obvious aggregation. When the volume fraction of (CO+H2) is constant, increasing the volume fraction of H2 is more conducive to the increase of the iron phase in the sinter.Compared with CO-CO2-N2, metal iron can be formed at the periphery and core of pellets at a lower temperature, and increasing the H2 volume fraction increases its porosity and surface area. In the study of the expansion behavior of pellets under different atmospheres, it was also found that there are some large particles of iron oxide that are not reduced during the reduction of CO, and the iron phase is small fragments after reduction. During H2 reduction, the reduction of iron oxide is relatively complete, no large particles of iron oxide are found, and the iron phase after reduction is relatively dense. This is because the reaction rate of H2 and iron oxide is fast, which promotes the formation of a large number of iron crystal nuclei, and the adjacent metal iron grains gather to form an iron layer, filling the cracks and pores caused by volume expansion, and making the pellet structure compact and shrink. This also enables H2 reduction to effectively inhibit the abnormal expansion caused by the growth of iron whiskers. According to the first principle calculation results based on density functional theory and the research results of molecular dynamics theory, when FeO is reduced by CO, the precipitated iron on the surface of FeO grows into a whisker structure, while when FeO is reduced by H2, the precipitated iron on the surface of FeO grows into a dense layered structure (Figure 2). The different morphologies of the iron precipitated during the reduction of FeO by CO and H2 are consistent with the macroscopic experimental results.
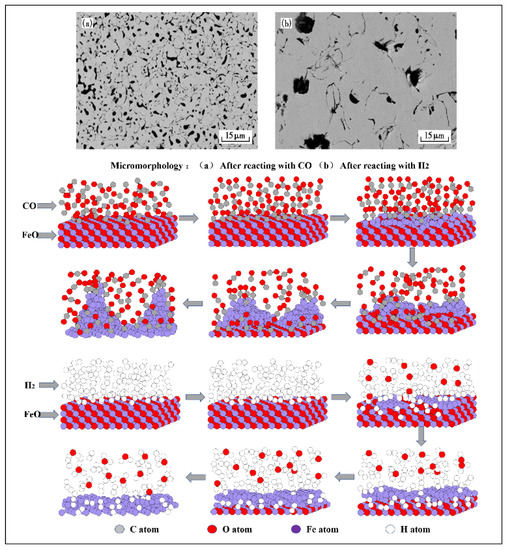
Figure 2. Microstructure of iron oxide reacting with CO and H2, adapted from [42,47]. (a) After reacting with CO; (b) After reacting with H2.
2. Effect of H2 on Gasification Behavior and Structure Evolution of Coke
The gasification reaction of coke is one of the important reasons for the deterioration of coke property. After hydrogen-rich smelting in the BF, H2 reduces iron ore to generate H2O, which gasifies with coke, thus changing the gasification reaction behavior and structure evolution process of coke in the BF. At present, researchers have conducted some research work on the gasification reaction behavior of H2O and coke. According to the literature, a comparative study on the thermodynamic and kinetic behaviors of the solution loss reaction of coke with CO2 and H2O found that the initial reaction temperature of coke with H2O is lower than that of coke with CO2 (Figure 3). The internal diffusion condition and interface reaction condition of coke gasification in H2O are better than that of CO2, and the difference in diffusion property is greater than that of interface reaction property. Moreover, the chemisorption capacity of CO2 on coke surface is weaker than that of H2O.The limiting link in the reaction with H2O is that the area of the interface reaction is larger than that of CO2 (Figure 4). Also, the higher the volume fraction of H2O, the greater the increase of the area controlled by the interfacial reaction with the increase in temperature.
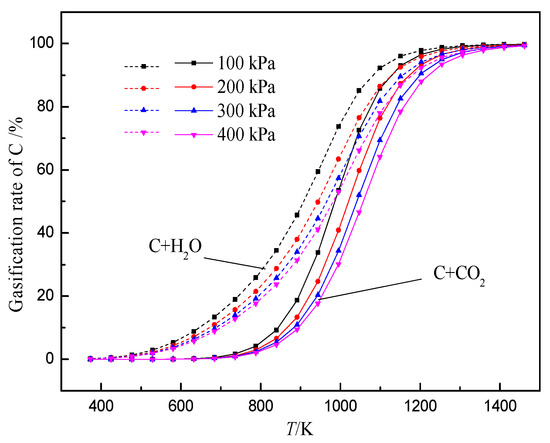
Figure 3. Relationships between gasification rate and temperature of carbon reacting with CO2 and H2O, adopted from [55], with permission from Elsevier 2018.
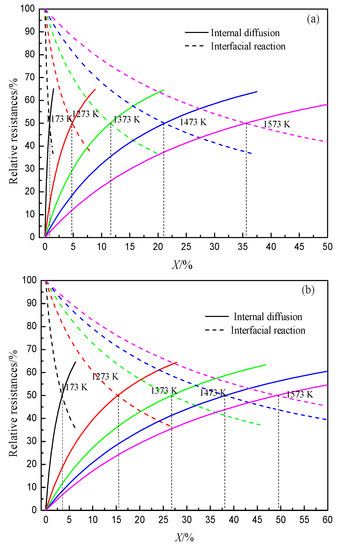
Figure 4. Relationship between reaction ratio and relative resistances at different temperatures, adopted from [49]. (a) Coke gasification by CO2; (b) Coke gasification by H2O.
Due to the great differences in the thermodynamic and kinetic behaviors of the reaction of coke with H2O and CO2, the pore structure of coke after the reaction also changes greatly. Research indicates that compared with the reaction of coke and CO2, the reaction of coke and H2O mainly occurs on the surface of particles. When coke reacts with CO2 and H2O, the large pores at the edge increase by 66.98% and 94.01%, respectively, compared with that before the reaction. When the solution loss rate of coke increases by 1%, the strength after reaction with CO2 and H2O decreases by 1.200% and 0.786%, respectively. The influence of H2O on the strength of coke is less than that of CO2. Xu et al. studied the gasification reaction and structural change of coke under an H2O/CO2 atmosphere and obtained the microstructure before and after the coke reaction under different conditions. As shown in Figure 5, the study pointed out that H2O has greater damage to the coke structure than CO2.
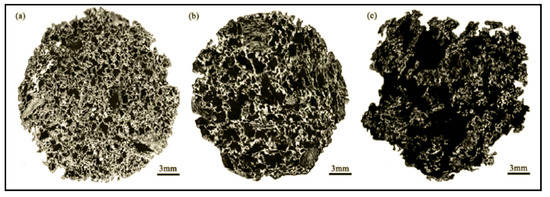
Figure 5. Panoramic images of coke under different reaction conditions, adopted from [64], with permission of American Chemical Society, 2017. (a) Unreacted coke; (b) Coke reacted with pure CO2; (c) Coke reacted with pure H2O.
In the BF, the iron oxide is reduced with rising high-temperature reducing gas (CO and H2) to generate a large amount of CO2 and H2O, which can react with coke to generate CO and H2 again. This shows that in the actual production process of BF, CO2, and H2O participating in the coke gasification reaction mainly come from the reduction of iron oxide, and the concentration depends on the reduction degree of iron oxide. This indicates that there is a coupling behavior between ore reduction and coke gasification in the BF (hereinafter referred to as “ore-coke coupling reaction”).The gasification reaction rate of H2O and coke is much higher than that of CO2 and coke. This indicates that the presence of H2 intensifies the gasification of coke (Figure 6), and with the increase of H2 volume fraction, the reduction of iron oxide tends to proceed in the low-temperature region, while the volume fraction of CO2 and H2O produced in the high-temperature region decreases. This increases the porosity of coke in the low-temperature zone but decreases the internal porosity of coke in the high-temperature zone (Figure 7), which helps to improve the strength of coke after a high-temperature reaction.
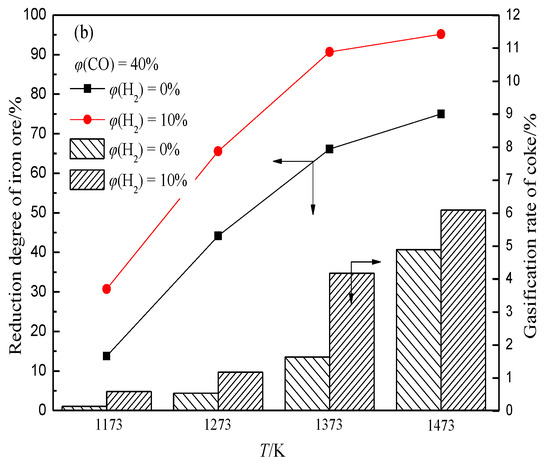
Figure 6. Effects of H2 on reduction degree of iron ore and coke gasification rate under different conditions, adopted from [67]. (a) φ(CO) = 30%; (b) φ(CO) = 40%.
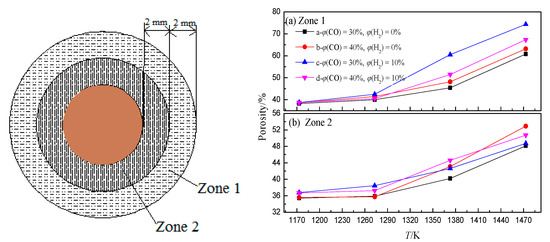
Figure 7. Porosity of coke zone 1 and zone 2 under different conditions, adopted from [67]. (a) Zone 1; (b) Zone 2.
3. Effect of H2 on Cohesive Zone of Blast Furnace
The softening and melting of the iron-bearing burden in the BF is mainly due to the gradual increase of wüstite mass fraction with the increase of temperature, which forms a large number of low melting point compounds with the minerals in the slag, and makes the slag and iron gradually separate and drip.Research has pointed out that the increase of H2 volume fraction in gas can accelerate the reduction of iron oxide, and a large amount of wüstite forms in the burden at a lower temperature.With the increase in temperature, the burden is rapidly reduced to metal iron, which changes the melting state of the slag and iron in the cohesive zone.With the increase of H2 volume fraction under an N2-CO-H2 atmosphere, the softening start temperature of burden decreases and the softening end temperature increases, which widens the softening temperature range, and the melting temperature range of slag iron decreases greatly, which improves the permeability of the cohesive zone (Figure 8).
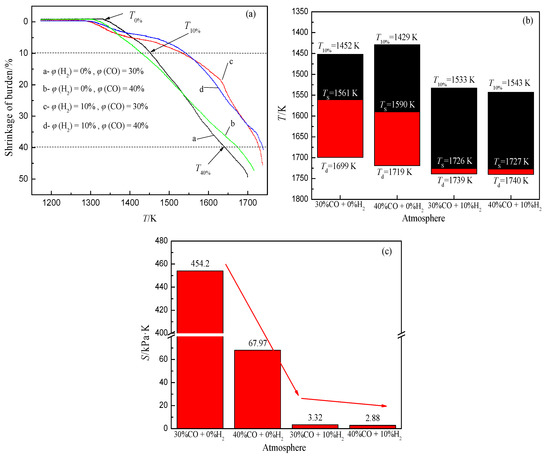
Figure 8. Change of softening-melting property of burden, adopted from [72], with permission from Elsevier, 2020. (a) Relationship between shrinkage and temperature; (b) Characteristic temperature of cohesive zone; (c) Permeability index of cohesive zone under different atmospheres.
**The article has been abridged. For the full text, please see "Effect of H2 on Blast Furnace Ironmaking: A Review"----Lan, C., Hao, Y., Shao, J., Zhang, S., Liu, R., & Lyu, Q. (2022). Effect of H2 on Blast Furnace Ironmaking: A Review. Metals, 12(11), 1864. https://doi.org/10.3390/met12111864
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




