Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere (1)
With the strong demand for production methods that could effectively counteract global warming and CO2 emissions, steelmaking production using electric arc furnaces (EAFs) is getting renewed attention. In order to prepare for the anticipated drastic growth of EAF-based steelmaking industries, it is necessary to find an alternative iron source to compensate for the shortage of high-quality scrap, which is currently the most important raw material for EAF-based steelmaking. Direct reduced iron (DRI), which was developed several decades ago, is a promising candidate. Recently, many studies have been conducted on the operational changes in the EAF process that would be needed to utilize DRI. A number of steelmaking companies with access to abundant natural gas have already been using a considerable amount of DRI in their EAF process. Since more demand for DRI is expected in the near future, more efficient production technology is required.
The production processes for DRI can be largely categorized into two types: gas-based processes and carbon-based processes. Although DRI production by gas-based processes has been more dominant than carbon-based processes, a large amount of hydrocarbon fuel, such as LNG, is required for direct reduction in gas-based DRI production. The reduction efficiency may considerably decrease unless the counter-current flow of the charges and reducing gas is properly established. Therefore, the carbon-based process can be favorable in case of a short supply of LNG and low efficiency of a reactor, such as a rotary kiln.
On the other hand, the mining amount of high-quality iron ore is currently lacking and its depletion will be encountered in the near future. Thus, innovative technologies for utilizing low-quality iron ore, such as magnetite, in DRI production are strongly required. Generally, solid carbonaceous materials such as coal are combined with iron ore into pellets for more efficient solid–solid reactions. At the initial stage of reduction by the composite carbon pellets, the emission of CO gas leaves a considerable number of pores in the pellet. If reducing gas is subsequently introduced, the porosity of the carbon-based reduction may enhance the mass transfer of gases and improve the net rate of the reduction reaction in the gas-based reduction. On the other hand, the melting point of reduced iron can be decreased by carburization when excessive carbon exists in the carbon-based reduction. Because molten iron may adhere to each other as well as refractories, the formation of liquid iron should be avoided in the direct reduction in rotary or shaft kilns.
Therefore, it is meaningful to seek optimized conditions for carbon-based reduction to complement gas-based reduction. In the present study, direct reduction experiments of iron ore pellets were carried out using hybrid gas-based reduction assisted by carbon-based reduction.
2. Materials and Methods
2.1. Sample Preparation
As raw materials for iron ore and coal, magnetite and anthracite, whose compositions are shown in Table 1, were chosen. Each material was pulverized in a disk mill and dried thoroughly in a box furnace at 383 K or a desiccator for more than 48 h. Afterwards, both materials were classified into several size groups using a series of sieves. To properly simulate the practical conditions of tailing magnetite fine particles, the iron ore and coal powder were classified into three size groups: 32–53 μm (S), 53–74 μm (M), and 74–105 μm (L). Using the analyses of optical microscopes, the average size of each group was estimated to be 43 μm, 64 μm, and 91 μm, respectively, and these average values were semi-quantitively verified based on the optical observation.
Table 1. Compositions of iron ore and coal used in the present study.
| Magnetite ore | t-Fe | Fe3O4 | Fe2O3 | MgO | SiO2 | CaO |
| 43.5% | 53.7% | 6.6% | 15.1% | 17.7% | 4.3% | |
| Anthracite | F.C. | V.M. | Ash | |||
| 58.9 | 6.5% | 34.7% |
Because the amount of anthracite coal obtained in the 53–74 μm group was not sufficient, only the other two size groups were used for the coal.
To determine the optimum conditions for the carbon composite as complementary pre-reduction, the carbon equivalent, which represents the molar ratio of supplied carbon from fixed carbon against oxygen to be reduced, were varied in the range of 0–1.0. A number of gas reduction experiments without carbon addition (CEq. = 0) were also performed for comparison. Magnetite ore and anthracite coal under various carbon equivalent conditions were carefully mixed and pressed at 3000 kg/cm2 using a pellet press into a disk-type carbon composite pellet with a diameter and height of 15 mm and 10 mm, respectively.
2.2. Experimental Procedure
The reduction experiments of the prepared pellets were carried out by analyzing the composition of the off-gas. A pellet in a quartz crucible (i.d. 22 mm, depth 27 mm) was placed in a bottom-closed quartz tube (i.d. 27 mm), which had both a gas inlet (o.d. 6 mm, i.d. 4 mm) and outlet at the top. The experimental apparatus used in this study is schematically shown in Figure 1. After a few hours of purging in a quartz tube with high-purity Ar gas as a carrier gas with 1360 cm3/min, the quartz tube holding the sample was inserted in a vertical electric resistance furnace controlled at 1373 K using Kanthal heaters. From the time when the sample was inserted into the furnace, the composition (CO, CO2, O2) of the off-gas was analyzed using a gas analyzer (NOVA9K, MRU, Neckarsulm, Germany). In order to calculate the amount of evolved CO or/and CO2 gases from dynamic gas compositions, as a tracer gas, O2 gas was directly introduced into the analyzer at a known flow rate, controlled to be 240 cm3/min by a mass flow controller at room temperature.
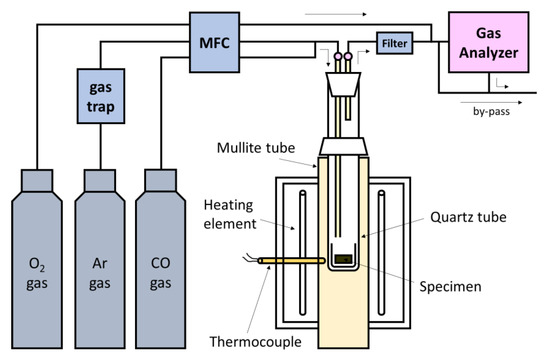
Figure 1. Schematic presentation of the experimental apparatus.
In the case of carbon composite reduction, only Ar gas was introduced, and the concentrations of CO and CO2 gas in the off-gas were analyzed to investigate the reduction behavior. The reduction was carried out until the emission of both CO and CO2 gas became negligible and was completed in 50 min in most cases. The gas reduction behavior could be determined from the amount of CO2 emitted during the introduction of the CO + Ar mixture gas into the quartz tube. Several experiments combining carbon-based and gas-based reduction were divided into two stages for the separate consideration of the two reduction mechanisms. In the first stage, CO and CO2 gases were detected by the composite carbon. After the evolution of CO and CO2 gases was completed, the carrier gas was switched to CO gas to simulate the gas reduction in the second stage. When CO2 gas evolved from the gas reduction decreased to a negligible level, the quartz tube was drawn out to be air-cooled.
To investigate the effect of pore formation, the true density of each sample after reduction was measured using an envelope density analyzer (GeoPyc 1360, Micromeritics, Norcross, GA, USA), while its apparent density was approximately estimated from the dimensions to obtain the porosity, as Equation (1)

The degree of reduction was determined as follows. The flow rates of the total gas from the sample could be obtained from the known flow rate of the tracer gas, O2 gas, and the composition of O2 gas (vol%) using a gas analyzer as shown in Figure 2. The relationship between the flow rates of the total gas and O2 gas is expressed by Equations (2) and (3). Subsequently, the flow rates of CO and CO2 gas from the reduction of the sample could be determined from that of the total gas, as shown below.

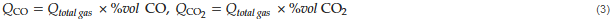
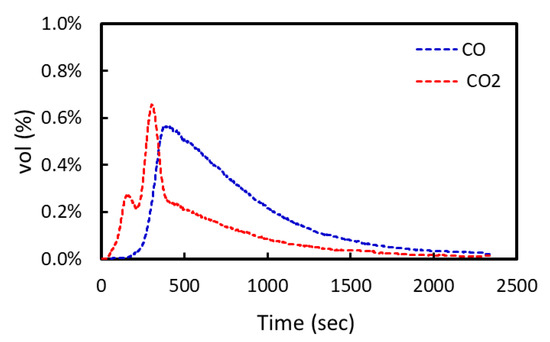
Figure 2. An example of the gas analysis during the reduction of iron ore by composite carbon.
Then, the integration of the flow rates of CO and CO2 gases with time represents the total amount of each gas from the reduction of the sample. Based on the accumulated amounts of CO and CO2 gases in each stage, the reduction degree, 𝑅𝑒, for the reduction by composited carbon and CO gas can be expressed as shown in Equation (4), respectively. Similarly, the reduction degree of the entire reaction can be estimated using Equation (5).
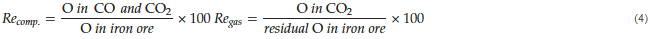

In addition to the reduction degree, as an index of residual carbon, the carbon efficiency for the reduction,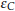 , could be further obtained for the whole reaction, as shown in Equation (6)
, could be further obtained for the whole reaction, as shown in Equation (6)
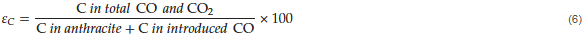
Song, S., & Kang, Y. Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere. Metals, 11(9), 1404. https://doi.org/10.3390/met11091404
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




