Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere (2)
3. Results and Discussion
3.1. Effect of Particle Size in Carbon-Based Reduction
Figure 3 shows the changes in the degree of reduction and carbon efficiency with different particle sizes. As the sizes of iron ore and coal particles decreased, the reduction reaction by carbon appeared to be promoted, which may be attributed to the larger surface area of the smaller particle size. This phenomenon indicates that as the number of contacts between magnetite ore and coal increases, the direct reduction reaction is more likely to occur than in the case with larger particle size.
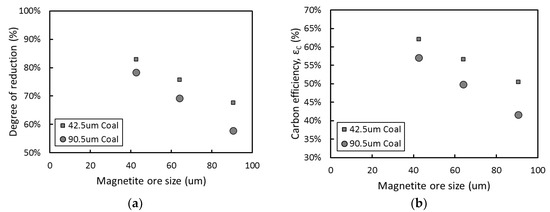
Figure 3. Reduction behaviors with various particle sizes: (a) degree of reduction (b) carbon efficiency.
It could be further verified by estimating the contacts between different kinds of particles, that is, iron ore and coal. A few decades ago, Norio and Tanaka developed a simple and effective expression as shown in Equation (7), based on a simple packing model, for the number of contacts between randomly mixed solid particles of different sizes.
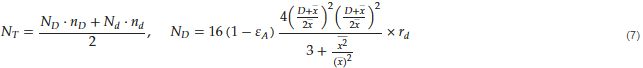
Here, NT and Ni stand for total number of contacts and the number of particles surrounding a particle i, while ni and ri the number and the fraction of a particle i, respectively. D, d, and 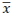 denote the diameters of larger and smaller particle and the average diameter, respectively. Moreover, εA refers to the surface porosity, which can be deduced from the volume porosity.
denote the diameters of larger and smaller particle and the average diameter, respectively. Moreover, εA refers to the surface porosity, which can be deduced from the volume porosity.
Using their model, the number of contacts between ore and coal particles was estimated to sharply increase when either the ore or coal particle size decreased, as shown in Figure 4. This phenomenon indicates that as the particle size decreases, the number of contacts between magnetite ore and anthracite increases, and a direct reduction reaction is more likely to occur than in the case with larger particle size. However, in Figure 5, it is shown that the relationship between the degree of reduction and the total number of contacts varies with the coal size.
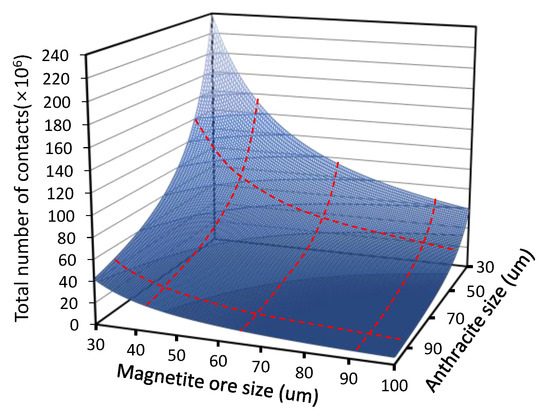
Figure 4. Change in the number of contacts between ore and coal with varying their sizes.
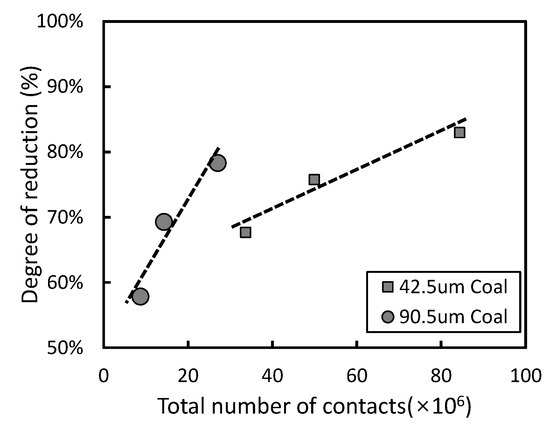
Figure 5. Relationship of the degree of reduction and the number of contacts.
The influence of the particle sizes of the iron ore and the carbon composite on the degree of reduction needs to be further clarified.
Recalling Figure 3, it can be also seen that the effect of the ore particle size on the reduction seems to be more significant than that of the coal particle size. Since the reduction characteristics can be also evaluated based on the porosity created by CO and CO2 gas evolution, the porosity of the reduced pellets was investigated. The effect of the size of iron ore and coal particles on the porosity of the reduced pellets is presented in Figure 6. As the ore particle size increased, the fraction of pores in the reduced pellets decreased. Contrary to the ore particle size, the coal particle size has a negligible influence on the porosity, which is essential for enhancing the subsequent reduction by gas. The pores can be formed by the evolution of CO and CO2 gas from the reduction of the ore. Whereas carbon sources for the gases are supplied from not only coal particles but also CO gas, only ore particles are able to provide oxygen for CO and CO2 gas formation. Therefore, pore formation appeared to be more dependent on the surface area of the ore particles than on that of the coal particles.
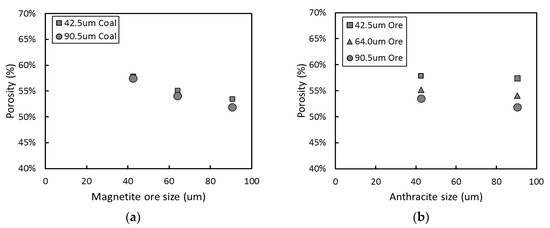
Figure 6. Relationship between porosity of the reduced pellets and particle size: (a) iron ore (b) coal.
3.2. Optimization of Carbon-Based Reduction Prior to Gas Reduction
To determine the optimum conditions for pre-reduction by composited carbon for better subsequent gas reduction, experiments for carbon-based and gas-based combined reduction were conducted using the smallest groups of ore and coal particles. Carbon with a certain equivalent was composited into iron ore pellets, and the reduction of the pellet by CO gas was initiated after the completion of the composited carbon reaction. Similar to the previous section, the degree of reduction was separately evaluated for the carbon-based and gas-based cases, as shown in Figure 7. Although the reduction mechanism shifts from gas-based reduction to carbon-based reduction as the carbon equivalent increases, which is not much different from expected, the overall degree of reduction adding up both the gas-based and carbon-based reduction did not change significantly.
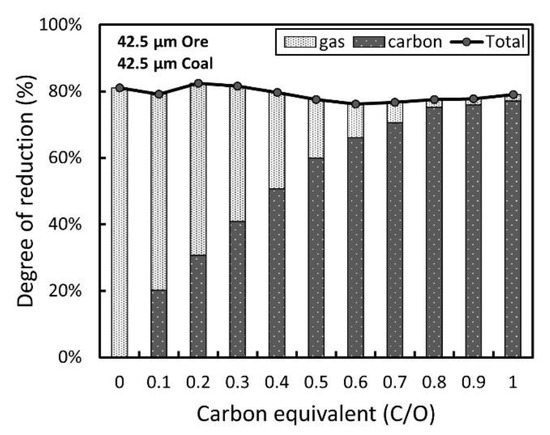
Figure 7. Relationship of the degree of reduction and the carbon equivalent.
Instead of the degree of reduction, the carbon efficiency could be used to establish the optimal conditions for maximizing the reduction efficiency by CO gas. Based on the amount of the emitted CO2 gas, the carbon efficiency of CO gas was estimated by varying the carbon equivalent. Figure 8 shows the influence of the carbon equivalent on the carbon efficiency of CO gas. For comparison, the efficiency of CO gas in the gas-only reduction (CEq. = 0) was calculated by integrating the CO2 gas emission from the point at which the degree of reduction by composited carbon in each carbon equivalent is equal to the degree of reduction during the gas reduction without carbon. In gas-only reduction without carbon, the carbon efficiency gradually decreases as the reduction progresses. On the other hand, the carbon efficiency of CO gas after the preliminary reduction was improved compared to the gas-only reduction. This improvement becomes evident when the carbon equivalent is greater than 0.2, possibly because of the formation of additional pores by the preliminary reduction.
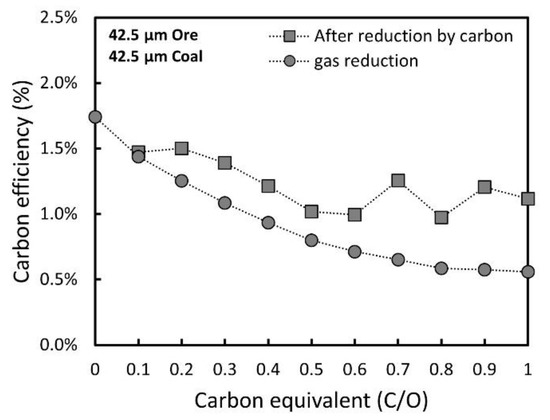
Figure 8. Changes in carbon efficiency of CO gas reduction with different carbon equivalents.
3.3. Kinetics of Reduction of Iron Ore by CO Gas
As discussed in the previous section, the efficiency of the reduction by gases could be improved by more pore formation, which can increase the mobilities of CO and CO2 gases. The effect of gas mobility is also meaningful in discussing the kinetics of the reduction reaction by the reducing gas. The kinetics of the reduction of iron ore by reducing gas has been intensively analyzed by many researchers. It has been found that the reduction behavior of iron ore by carbon or carbonaceous gas is strongly dependent on process conditions such as temperature, pressure, size of the solid particles, and pore structure. Because the entire reduction process is divided into several reaction steps, the rate of the total reduction reaction should be governed by a rate-controlling step.
There are typical equations for expressing the reduction reaction rate, which have been proposed to be related to specific rate-controlling steps, i.e., Boudouard reaction, topochemical reaction, and gas diffusion. The dominant reaction mechanism of reduction by gas in the present study was determined using known rate equation functions for each determining step. As shown in Figure 9, a simple comparison of the linearity of the functions with the present results indicated that the reduction by gas is mainly governed by gas diffusion over a wide range of carbon equivalents. The rate equation function for gas diffusion can be written in a simplified form as Equation (8), whose slope, K, is known to include the effective counter-current diffusivity of CO and CO2. Thus, the reduction kinetics can be discussed using the slope against the reaction time. In particular, the initial slope in the earliest stage should be considered to properly evaluate the effect of the pores formed by preliminary reduction.
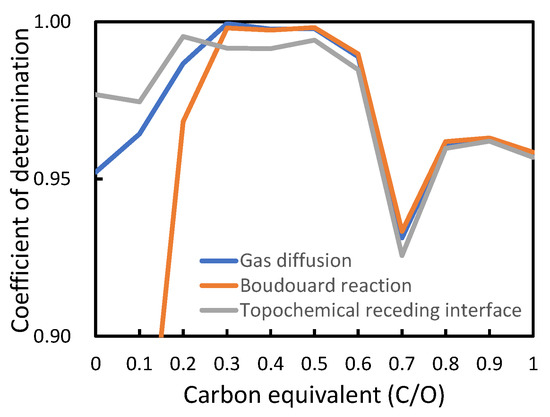
Figure 9. Comparison of the validity of various kinetic models in analyzing reduction process.

As shown in Figure 10, at a low carbon equivalent, the initial effective diffusivity of CO and CO2 gases increases with the increment of the carbon equivalent. It can be expected that more pores with more composited carbon enabled faster diffusion of CO and CO2 gases. When the carbon equivalent increases above 0.7, the gas mobilities of CO and CO2 are slightly reduced, because the reduction by CO gas is limited by an insufficient amount of unreduced residual ore. Hence, it can be concluded that a carbon equivalent of 0.5~0.7, seems to be a favorable condition to maximize the efficiency of the reducing gas under the present experimental conditions, based on the reaction rate and carbon efficiency.
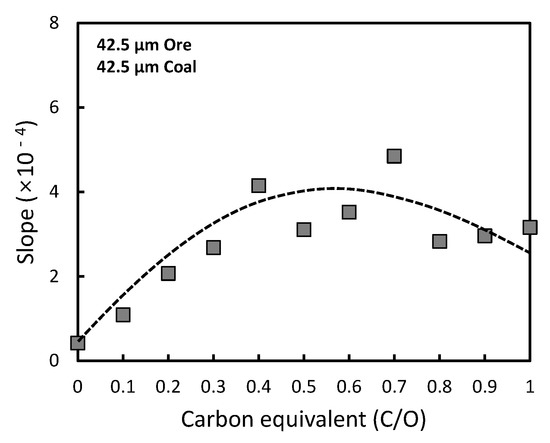
Figure 10. Relationship between effective diffusion coefficient and carbon equivalent.
It should be noted that preliminary reduction by carbon and subsequent reduction by gas cannot be clearly separated under actual conditions in industrial kilns. A reducing gas atmosphere (considerable CO partial pressure) may suppress the preliminary reduction by carbon in the early stages. Experiments on the hybrid reduction of iron ore were carried out using carbon and CO gas simultaneously. Since it was not possible to determine the degree of reduction by analyzing CO and CO2 gas in the off-gas, the metallization ratio of the pellets after interrupting the reaction at a certain time was quantified from the concentrations of metallic Fe, Fe+2, and Fe3+. The metallization ratios of the pellets are shown in Figure 11. Compared to the pellets interrupted similarly during the reduction by CO gas, the metallization ratio of pellets by combined reduction is substantially higher, which means that the reduction rate of the combined reduction by carbon + CO gas is superior to that of the gas reduction only. It was verified that the present hybrid reduction can be applied to even industrial conditions using reducing gas.
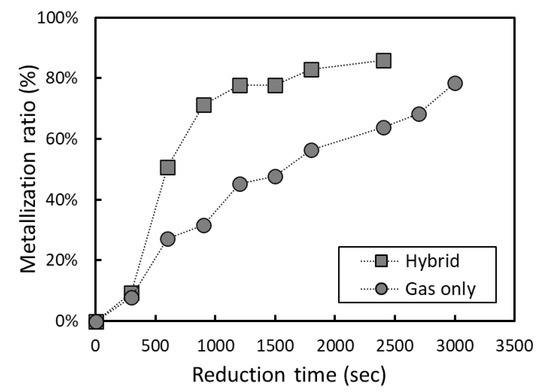
Figure 11. Comparison of metallization ratio for combined reduction and reduction by CO gas.
Conclusively, it is expected that the consumption of hydrocarbon fuel for DRI production can be cut down because the reduction is completed in a shorter time, as shown in Figure 11. Hence, the hybrid DRI production process is possible with less CO2 emissions. As explained in Section 3.2, moreover, the efficiency of reducing gas could be improved by the preliminary reduction using composited carbon, resulting in further reduction of CO2 emission. The overall effect of the hybrid reduction of iron ore may be quantitatively analyzed in further study.
4. Summary
The feasibility of combining carbon composite reduction and gas reduction was experimentally investigated. It was expected that pre-reduction by the composite carbon could improve pore formation and gas reduction. First, the fundamental conditions for carbon-based reduction to form more pores were sought. When the particle size decreased, the number of contacts between the iron ore and coal particles increased, and the degree of reduction and carbon efficiency increased, leaving more pores in the pellet. It was also found that ore particle size has a greater influence on pore formation than coal particle size. In the carbon-CO gas 2-step reduction experiments with a controlled carbon equivalent, the efficiency of gas reduction was enhanced by the preliminary reduction by carbon composited with a carbon equivalent greater than 0.2. The analysis using the reduction kinetics showed that the reduction rate is dependent on the diffusivity of CO and CO2, which can be maximized at a carbon equivalent of 0.5~0.7. Finally, the applicability of the combined reduction to industrial practice was confirmed based on a higher reaction rate.
Song, S., & Kang, Y. Effect of Carbon Addition on Direct Reduction Behavior of Low Quality Magnetite Ore by Reducing Gas Atmosphere. Metals, 11(9), 1404. https://doi.org/10.3390/met11091404
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




